Abiocode Logo
Products
Contact Us
- Telephone:
1-818-707-0309 - E-Mail:
Abiocode@Abiocode.com
R3672-2 - OSBPL8 (C) Antibody, Rabbit Polyclonal
|
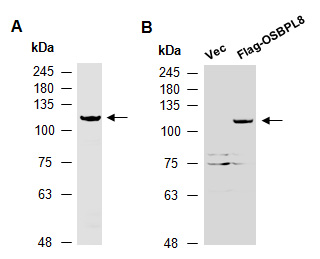
Quantity: 100 ul Application: WB Predicted I Observed M.W.: 101 I 120 kDa Uniprot ID: Q9BZF1
Background: Oxysterol-binding protein-related protein 8 (OSBPL8) is a member of the oxysterol-binding protein (OSBP) family, a group of intracellular lipid receptors. Like most members, OSBPL8 contains an N-terminal pleckstrin homology domain and a highly conserved C-terminal OSBP-like sterol-binding domain. OSBPL8 is involved in lipid countertransport between the endoplasmic reticulum and the plasma membrane: specifically exchanges phosphatidylserine with phosphatidylinositol 4-phosphate (PI4P), delivering phosphatidylserine to the plasma membrane in exchange for PI4P, which is degraded by the SAC1/SACM1L phosphatase in the endoplasmic reticulum. Other Names: Oxysterol-binding protein-related protein 8, ORP-8, OSBP-related protein 8, KIAA1451, ORP8, OSBP10, MST120, MSTP120 Source and Purity: Rabbit polyclonal antibodies were produced by immunizing animals with a GST-fusion protein containing the C-terminal region of human OSBPL8. Antibodies were purified by affinity purification using immunogen. Storage Buffer and Condition: Supplied in 1 x PBS (pH 7.4), 100 ug/ml BSA, 40% Glycerol, 0.01% NaN3. Store at -20 °C. Stable for 6 months from date of receipt. Species Specificity: Human, Mouse Tested Applications: WB: 1:500-1:2,000 (detect endogenous protein*) *: The apparent protein size on WB may be different from the calculated M.W. due to modifications. Product Data:
|